About us
At Documedex, we are dedicated to guiding medical device manufacturers and developers through the complex world of regulatory compliance. With a deep understanding of the global medical device regulatory landscape, we help you navigate the stringent requirements to bring your products to market efficiently and safely.
Our team consists of seasoned technical experts, clinicians, experienced regulatory affairs professionals and Quality Assurance specialists who have worked with medical device manufacturers for over 20 years. They bring extensive experience in regulatory affairs, technical documentation requirements, quality management systems, clinical evaluation, and risk management.
Our Mission
We are committed to empowering companies by offering tailored, expert-driven solutions that not only ensure compliance but also foster innovation and growth in the medical device industry. Our services include consulting in:
- Regulatory Pathway
- Technical Documentation according to EU MDR 2017/745
- CE Certification (EU MDR 2017/745)
- ISO 13485 & EU MDR Quality Management System
- MDSAP (Medical Device Single Audit Program - Australia/ USA/ Japan/ Brazil/ Canada)
- Clinical Evaluation and Clinical Trial
- Risk Management according to ISO 14971
- Usability Study (IEC 62366-1 / EN 62366-1)
- Software Validation (IEC 62304 - SaMD)
- Registration of medical device globally (EUDAMED, ARTG, etc)
- Providing templates relevant to Technical Documentation according to EU MDR 2017/745 (CE Marking) and Quality management System (ISO 13485, EU MDR, MDSAP, etc)

Regulatory Pathway
Navigating the complex regulatory landscape for medical devices can be challenging. At DOCUMEDEX, we specialize in guiding medical device manufacturers through every step of the regulatory process, ensuring compliance with the latest regulatory requirements and standards and accelerating time-to-market.
Our Services Include:
1. Regulatory Strategy Development:
We assess your product’s classification and develop a customized regulatory strategy that aligns with your business goals and market targets, whether in the USA, Europe, Australia or global markets.
2. Regulatory requirements and documentation:
We assist in preparing the list of applicable standards, required pre-clinical and clinical studies to meet the regulatory requirements
3. Quality Management Systems (QMS) Requirements:
We inform you about the applicable requirements in compliance with ISO 13485 and applicable regulatory requirements (EU MDR, TGA, US FDA, etc.) relevant to your product and your scope based on the target markets.
4. Planning:
We assist you to provide a realistic regulatory compliance plan including the timeline for each step in the process.

Consulting for
CE Certification (EU MDR 2017/745)
We provide comprehensive consulting services to support medical device manufacturers in achieving EU MDR certification (CE Certification) and preparing technical documentation that meets stringent regulatory requirements.
Our EU MDR Certification Consulting Services
- Regulatory Pathway Guidance
- Gap Analysis
- Technical Documentation Preparation
- Quality Management System (QMS-MDR) Alignment
- Notified Body Preparation
Technical Documentation Services
- Technical File Development
- Medical Device description
- GSPR checklist preparation
- Consulting for Pre-clinical studies including required test reports
- Design verification and validation
- Risk management (ISO 14971)
- Usability Study (EN 62366-1)
- Software Validation (IEC 62304)/ Sterilization validation/ Shelf-life Study (If applicable)
- Biological Evaluation (ISO 10993 series)
- Clinical Evaluation Report (CER)
- Post-Market Surveillance (PMS) and PSUR
- Post Clinical Follow up (PMCF)
- Labelling and UDI Compliance
- EU Representative & Declaration of Conformity (DOC)
- etc.
Why Choose Us?
- Expertise in EU MDR: Extensive knowledge of the regulatory landscape and requirements for successful certification including Technical and clinical experts who have experiences as Assessor, auditor and clinician in Notified Bodies.
- Tailored Solutions: Customized strategies to fit your device type, risk classification, and market needs.
- End-to-End Support: From initial gap analysis to Notified Body interaction, achievement of CE Certificate and post-market compliance.
Our services ensure your technical documentation is complete, compliant, and ready for MDR certification, enabling smooth market access in the European Union.
Consulting for
Quality Management System (ISO 13485/ EU MDR/ MDSAP
We specialize in providing comprehensive consulting services tailored to medical device manufacturers seeking compliance with global quality management standards and regulations. We simplify the complex processes involved in implementing ISO 13485, MDSAP, and MDR-QMS, enabling manufacturers to focus on innovation while ensuring compliance and market readiness. Our services include:
Gap Analysis
- Assess processes against ISO 13485 and/or MDSAP and/or EU MDR-QMS requirements.
- Identify deficiencies and provide a roadmap for compliance.
Custom QMS Design
Develop a tailored QMS, including policies, procedures, and work instructions.
Documentation Development
Create quality manuals, process documents, and templates required for ISO 13485 and/or MDSAP and/or EU MDR-QMS compliance.
Implementation Support
Streamline key processes such as design control, risk management, and supplier qualification.
Train your staff to ensure a culture of compliance.
Audit and Certification Support
Conduct pre-certification internal audits and resolve non-conformities.
Facilitate communication with certification bodies.
Why Choose Us?
- Global Expertise: Proven track record in helping manufacturers comply with ISO 13485, MDSAP, and MDR across multiple markets.
- Tailored Solutions: Custom implementation plans that suit your business size and resources.
- End-to-End Support: From initial assessments to certification and beyond.
- Regulatory Insight: In-depth understanding of regulatory frameworks for FDA, TGA, EU MDR, HC, and others
Download Templates
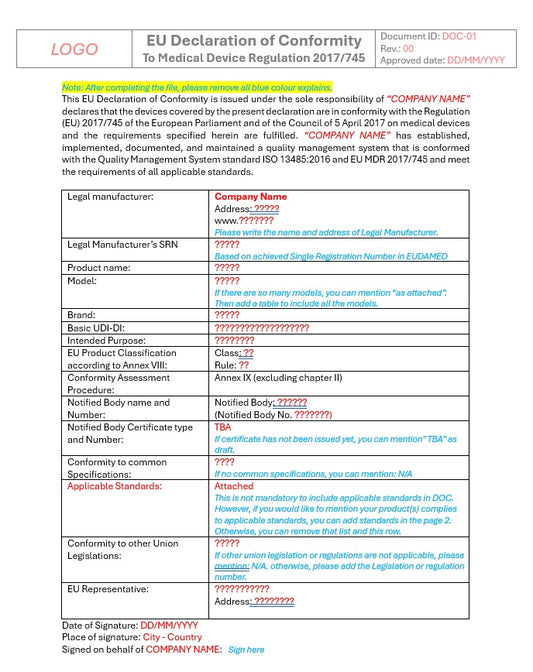
EU MDR 2017/745 Technical Documentation

Consulting services for Completing the templates
Our consulting team including Assessors and Clinicians who have experience of working with Notified Bodies, offers expert guidance to help you efficiently complete and tailor our EU MDR technical documentation templates to meet your specific product and regulatory requirements. We ensure compliance with the latest EU MDR standards and Notified Body expectations.
Our Services Include:
- Template Customization
Assistance in adapting the templates to match your medical device type and classification.
Tailored solutions for specific technical documentation needs.
Regulatory Compliance Review (Pre-Assessment)
Validation of content to ensure it complies with EU MDR.
Alignment with Notified Body and Competent Authority expectations.
Gap Analysis and Feedback
Review of the completed documents to identify gaps or non-compliance risks.
Document Completion Support
Step-by-step guidance on completing all sections of technical documentation e.g. General Safety and Performance Requirements (GSPR), Pre-clinical studies, Design Verification and validation, Label& IFU, Clinical Evaluation Report (CER), PMS& PSUR, PMCF, Software validation, Sterilization validation, stability study, Usability study, Risk Management File, etc.
Training and Support
Training sessions for your team on how to effectively use and complete the templates.
Ongoing support during the document preparation and submission process.
Why Choose Us?
- Expertise: Over 20 years of experience with Notified Bodies, regulatory agencies, and medical device manufacturers.
- Efficiency: Save time with professional guidance to reduce back-and-forth with regulatory bodies and reduce the costs relevant to Notified Body Assessments.
- Compliance: Ensure your documentation aligns with the EU MDR and withstands scrutiny.
- Obtaining CE Certificate: Certification process in a correct and effective direction