Template of
Declaration of Conformity
Declaration of Conformity
Free Download
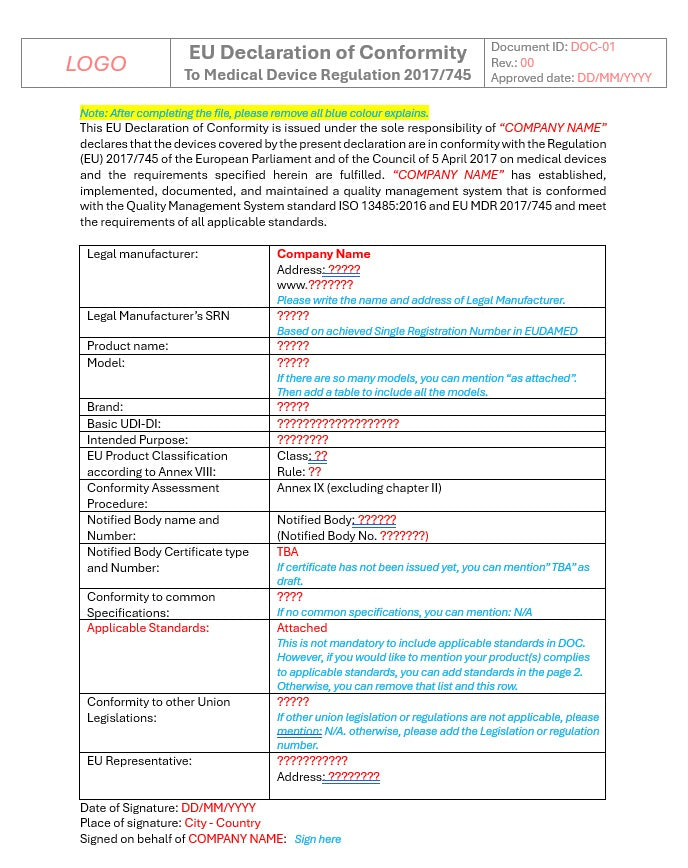
Ensure your medical device is compliant with the European Union Medical Device Regulation (EU MDR 2017/745) by using our Declaration of Conformity Template. This professionally designed template simplifies the process of declaring your device’s conformity with all applicable regulatory requirements, providing a ready-to-use solution for manufacturers, and regulatory teams.
Key Features:
- Fully EU MDR 2017/745 Compliant: The template is aligned with Article 19 and Annex IV of the EU MDR, ensuring all necessary declarations and references to standards are covered.
- Structured for Easy Completion: The template provides clearly defined sections to include essential information such as device identification, manufacturer details, applicable standards, and conformity assessment procedures.
- Customizable for All Device Classes: Whether your device is classified as Class I, IIa, IIb, or III, this template is adaptable to various types of medical devices, ensuring compliance regardless of the risk classification.
- References to Relevant Harmonized Standards: Space to list relevant harmonized standards and regulations, allowing you to provide evidence of compliance with the General Safety and Performance Requirements (GSPR).
- Flexible Formats: The template is fully editable, allowing customization based on your device’s specific characteristics and models
Why Choose This Template?
- Regulatory Assurance: Aligned with Article 19 and Annex IV of the EU MDR, ensuring your Declaration of Conformity is correctly structured and covers all necessary elements.
- Save Time & Effort: Eliminate the need to create this crucial document from scratch. Our template speeds up the process while ensuring it adheres to the required format.
- Adaptable for All Device Types: Suitable for manufacturers of all medical device classes, including those requiring Notified Body involvement.
- Audit-Ready Documentation: Ensures your documentation is correctly structured and traceable, making it easy for you to demonstrate compliance during regulatory audits.
What’s Included?
- Editable Template in Word format: Customizable document to include specific device and manufacturer information.
- Guidance Notes: Step-by-step instructions and example for completing each section of the declaration.
- Pre-Structured Sections: All mandatory elements, including device identification, manufacturer’s details, relevant EU legislation, conformity assessment procedures, and more.
Ensure Compliance with EU MDR 2017/745
Our Declaration of Conformity Template simplifies the process of demonstrating that your medical device meets the requirements of EU MDR 2017/745. Whether you are preparing for initial certification or maintaining compliance for recertification, this template provides all the necessary elements for a complete, accurate, and audit-ready Declaration of Conformity.
Share